Polyethylene terephthalate (PET): A brief introduction
PET is a very common plastic, mostly encountered in our lives as PET bottles and as a food packaging material. In this article you will learn how NIR spectroscopy can improve the efficiency of your PET analysis at different steps along the production cycle. Before getting into this, let’s introduce some background information about PET.
Polyethylene terephthalate (PET)
Polyethylene terephthalate (PET) is a general-purpose thermoplastic polymer which belongs to the polyester family. Polyester resins are known for their excellent combination of properties such as mechanical, thermal, and chemical resistance as well as dimensional stability.
PET is one of the most recycled thermoplastics and has the number 1 as its recycling symbol. Recycled PET can be converted into fibers, fabrics, sheets for packaging and for manufacturing automotive parts. PET is a highly flexible, colorless, and semi-crystalline resin in its natural state. Depending upon how it is processed, it can be semi-rigid to rigid. It exhibits good resistance to impact, moisture, alcohols, and solvents.
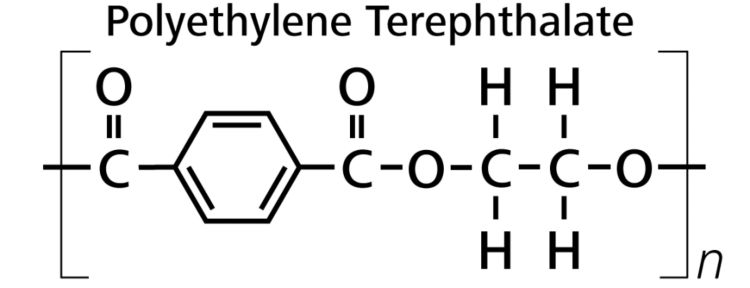
The chemical formula of PET is (C10H8O4)n and its molecular structure is shown in Figure 1.
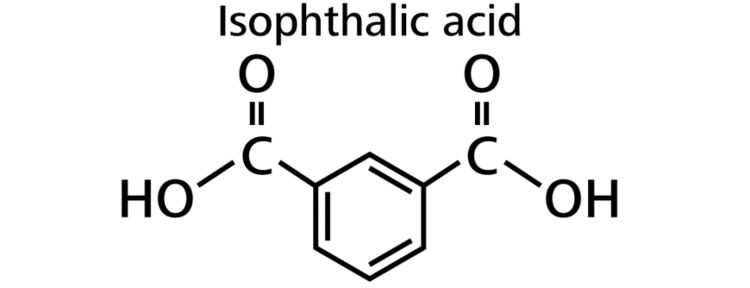
In addition to linear PET, there is also a branched version of the polymer. Branched PET is typically mixed with a small percent of isophthalic acid (C8H₆O4), because purified isophthalic acid (PIA, Figure 2) reduces the crystallinity of PET, serving to improve its clarity and increase the productivity of bottle manufacturing processes.
Diethylene glycol (DEG) as an additive also reduces the rate of crystallization of PET when crystallizing from the melt, isothermally and dynamically.
The key properties and advantages of PET resin are numerous:
- very strong and lightweight, and therefore easy and efficient to transport
- has good gas (oxygen, carbon dioxide) and moisture barrier properties, meaning low gas permeability (particularly against CO2)
- exhibits excellent electrical insulating properties
- broad range of use temperature (-60 to 130 °C)
- high heat distortion temperature (HDT)
- suitable for transparent application purposes
- practically shatter-resistant – PET does not break or fracture and is used to replace glass in some applications
- recyclable material
- transparent to microwave radiation
- very resistant to alcohols, aliphatic hydrocarbons, oils, greases, and diluted acids
- moderately resistant to diluted alkalis, aromatic and halogenated hydrocarbons
- PET is approved as safe for contact with foods and beverages by the FDA, Health Canada, EFSA, and other health agencies
What is polyethylene terephthalate (PET) used to make?
Polyethylene terephthalate is used in several types of packaging applications as shown in Figure 3. Due to its strength, light weight, and many other attractive properties, PET excels as a food packaging material.

Polyester makes up nearly two-thirds of synthetic fibers produced. There are many different types of polyester, but the type most often produced for use in textiles is PET. When used in a fabric, it is most often referred to as «polyester» or «poly» (Figure 4). This material costs very little to produce, which is the primary driver for its use in the textile industry.
Approximately 60% of the global PET production is used to make fibers for textiles while about 30% is used to make bottles for various purposes. Its ability to be recycled is especially attractive for manufacturers looking to save costs and operate in a greener manner.

In the electronics industry, PET is chosen to replace less ideal materials due to its excellent electrical insulating properties and resistance to distortion even at high temperatures. PET is also used to manufacture many parts in the automotive industry (Figure 5).
NIRS as a tool to assess the quality of PET
For over 30 years, near-infrared spectroscopy (NIRS) has been an established method for fast and reliable quality control within the PET industry. Despite this, many producers still do not consistently consider the implementation of NIRS in their QA/QC labs. Limited experience regarding application possibilities or a general hesitation about implementing new methods are some of the reasons behind this.
The advantages of using NIR spectroscopy for QA/QC are numerous. One major advantage of NIRS is the determination of multiple parameters in just 30 seconds with no sample preparation! The non-invasive light-matter interaction used by NIRS, influenced by physical as well as chemical sample properties, makes NIRS a suitable method for the determination of several critical quality parameters in these polymers and many more.
In the remainder of this article, a short overview of PET applications is presented, followed by available turnkey solutions for PET, developed according the NIRS implementation guidelines of ASTM E1655.
For more detailed information about NIRS as a secondary technique, read our previous blog posts on this subject.
Applications and parameters for PET with NIRS
During production of PET it is important to check certain parameters to guarantee the quality. These parameters include the diethylene glycol content, isophthalic acid content, intrinsic viscosity (ASTM D4603), and the acid number (AN). Determination of these parameters is a lengthy and challenging process due to the limited solubility of the sample and the need to use different analytical methods.
The most relevant applications for NIRS analysis of PET are listed in Table 1.
Table 1. Available Application Notes for use of NIRS for PET
| Polymer | Parameter | Related NIRS Application Notes |
|---|---|---|
| Polyethylene terephthalate (PET) | Diethylene glycol, Intrinsic Viscosity, Acid number, Isophthalic acid | AN-NIR-023 |
Where can NIRS be used in the production process of PET?
Figure 6 shows the individual production steps from the plastic producer via plastic compounder and plastic converter to the plastic parts producer. The first step in which near-infrared lab instruments can be used is when the pure polymers like PET are produced, and their purity needs to be confirmed. NIRS is also a very useful technique during the next step where polymers are compounded into intermediate products to be used for further processing.
Easy implementation of NIR spectroscopy for plastic producers
Metrohm has extensive expertise with analysis of PET and offers a turnkey solution in the form of the DS2500 Polymer Analyzer (Figure 7). This instrument is a ready-to-use solution to determine multiple quality parameters in PET.
Application example: Pre-calibrations available for the PET industry on the DS2500 Polymer Analyzer
Due to the limited solubility of polyethylene terephthalate and the need to use several different analytical methods, the determination of the parameters listed in Table 2 is a lengthy and challenging process with conventional laboratory techniques.
| Parameter | Primary method | Time to result (primary method) | NIRS benefits |
| Diethylene Glycol content | Extraction + HPLC-MS |
45 min. preparation + 40 min. HPLC-MS |
All four parameters are measured simultaneously within a minute, without sample preparation or the need of any chemical reagents |
| Isophthalic acid content | Dissolve + HPLC | 45 min. preparation + 40 min. HPLC | |
| Intrinsic viscosity | Dissolve + Viscometer | 90 min. preparation + 1 min. Viscometer | |
| Acid number | Dissolve + Titration | 90 min. preparation + 10 min. Titrator |
The NIRS prediction models created for PET are based on a large collection of real product spectra and is developed in accordance with ASTM E1655 Standard practices for Infrared Multivariate Quantitative Analysis. For more detailed information on this topic, download the free White Paper.
White Paper: Near-Infrared Spectroscopy: Quantitative analysis according to ASTM E1655
To learn more about pre-calibrations for PET, download our brochure and visit our dedicated webpage.
The result of this turnkey solution for rapid non-destructive determination of the key quality parameters for PET listed in Table 2 is shown in Figure 8.
This solution demonstrates the feasibility of NIR spectroscopy for the analysis of multiple parameters in PET in less than one minute without sample preparation or using any chemical reagents. Learn more about the procedure in our free Application Note:
Other installments in this series
This article is a detailed overview of the use of NIR spectroscopy as the ideal QC tool for the analysis of polyethylene terephthalate (PET). Other installments in this series are dedicated to:
Overview of NIRS in polymer production
Your knowledge take-aways
More information about spectroscopy solutions provided by Metrohm
 Sdílet článek
Sdílet článek
