In Part 2 of this series on trace metal analysis with solid-state electrodes, we introduced the scTRACE Gold electrode. The third part of this series explains even more applications which can be performed with this electrode, but this time after modifying the gold micro-wire with a thin layer of another metal.
Why modify the electrode material?
As explained for the Bi drop electrode in Part 1 of this series, stripping voltammetry is a two-step measurement.
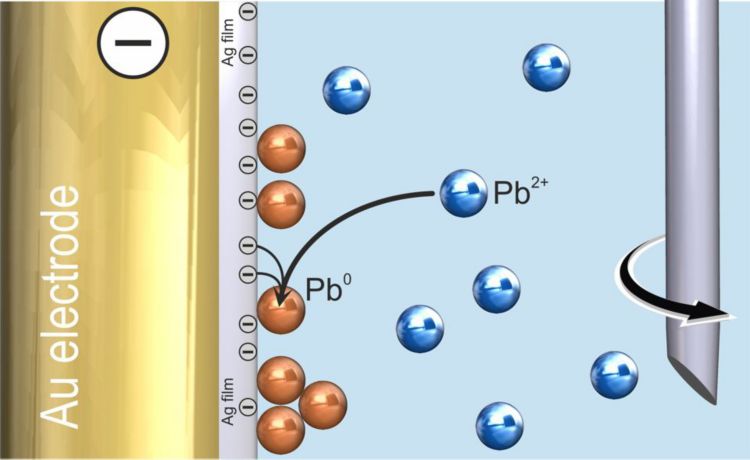
In the first step, the analyte is deposited on the working electrode. In the case of anodic stripping voltammetry (ASV), the analyte is reduced and forms an alloy with the electrode material (Figure 1). In the case of adsorptive stripping voltammetry (AdSV), the analyte forms a complex which is adsorbed to the working electrode.
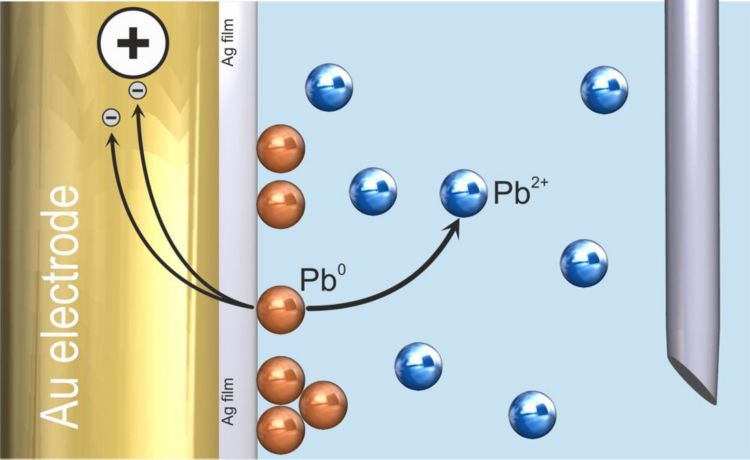
In the subsequent stripping step, the deposit is brought back into solution, giving the analytical signal which is proportional to the deposited amount of analyte. In the case of ASV, the electrochemical reaction is the re-oxidation of the analyte during an anodic scan (Figure 2). In the case of AdSV, the adsorbed metal complex is reduced during a cathodic scan.
Both steps, deposition as well as stripping, are subject to the principles of kinetics and thermodynamics. Without going into detail, the result is simply that some analytes cannot be determined with certain electrode materials. One way to solve this problem is to modify an existing working electrode with a different material that is more suitable.
Applications

Lead in drinking water
Most of the lead which is present in surface and ground water is of anthropogenic origin, resulting from the leaching of contaminated soils. Lead in tap water, however, often originates from the household plumbing system. Pipes from lead metal were popular in some countries until the 1970s. Although Pb is barely soluble in water, it slowly dissolves in the presence of oxygen. As a result, the allowed limit for lead in tap water can be easily exceeded by a significant amount. Now lead pipes for municipal water transport are forbidden, but there are still houses with old installations intact.
The WHO (World Health Organization) recommends a limit value for lead in drinking water of 10 µg/L. In the European Union, the upcoming limit is as low as 5 µg/L.

For the determination of lead, the scTRACE Gold electrode is modified with a silver film. The film is plated ex situ from a separate plating solution. Once plated, it can be used for multiple determinations. When the film is depleted, it can be removed, and then a fresh film is plated again. A side effect of the silver film is that the scTRACE Gold electrode lasts longer, since aging processes mainly affect the renewable silver film.
In a repeatability study, determining 10 µg/L Pb with three different electrodes on four different days (total number of determinations = 10) the average recovery of Pb was 96% with a relative standard deviation of 5%.
Using the 884 Professional VA it is possible to measure lead concentrations in water down to 0.4 µg/L, allowing a simple and reliable determination of even the future limit in the European Union.

With the 946 Portable VA Analyzer, the limit of detection is only slightly higher: 0.6 µg/L. However, the mobile use offers the possibility for close monitoring of individual installations without the need to preserve samples and send them in to a central lab. Furthermore, the concerned resident gets an immediate result on the spot.
Read our related Application Note below for more details.

Nickel and cobalt in drinking water
Similar to lead, nickel concentrations present in water sources can be increased by human influence as well. Plumbing fixtures and faucets are often plated with a thin layer of nickel for protection against corrosion, even if the finish is made of chromium. Furthermore, nickel is part of many alloys from stainless steel to nickel brasses and bronze. Nickel steel alloy cookware or nickel pigmented dishes can also cause increased nickel levels. The maximum allowed level in drinking water in the European Union is 20 µg/L whereas the WHO recommends a limit of 70 µg/L.
For the voltammetric determination of nickel and cobalt, an ex situ plated bismuth film on the scTRACE Gold electrode is used as working electrode. Nickel as well as cobalt are determined in the form of their DMG (dimethylglyoxime) complex. This method had already proven its reliability with the mercury electrode, and therefore this application can now be transferred to a mercury-free electrode. With a detection limit of 1 µg/L with the 946 Portable Analyzer, and even lower at 0.2 µg/L with the 884 Professional VA lab instrument, the method is surely sufficient to monitor the compliance with legal requirements. The recovery for 1 µg/L Ni in a standard solution is about 99% (mean of 10 determinations) with a relative standard deviation of 5%.
Click below to download the free related Application Note.

Chromium(VI) in drinking water
The problem of chromium(VI) in drinking water was brought to the attention of the general public with the movie «Erin Brockovich» in 2000, starring Julia Roberts. The plot is based on a true story, which happened in the small community of Hinkley, California, where the local energy provider contaminated the groundwater with the carcinogenic hexavalent chromium. The company attempted to cover up the incident, but an increased number of tumors and other health problems among the residents could finally be traced back to the contaminated drinking water.
Contamination with Cr(VI) in the environment is usually the result of improper handling of various industrial processes, especially abandoned waste dumped from galvanic chromium plating. The WHO recommends a maximum limit of 50 µg/L total chromium for drinking water.
After modifying the scTRACE Gold electrode with an ex situ plated mercury film, chromium(VI) can be determined as a complex with DTPA (diethylenetriaminepentaacetic acid). The recovery of a standard containing 30 µg/L Cr(VI) is 115% (mean of 3 determinations) with a relative standard deviation of 2%. Using the 946 Portable VA Analyzer it possible to determine concentrations down to 2 µg/L Cr(VI), allowing the on-site determination, providing immediate results without delay.
Download our free Application Note below to learn more.

Summary
Talking about all of the applications that are possible with the scTRACE Gold electrode (Figure 3) would go beyond the scope of this blog. The table below gives an overview of several elements for which methods with the scTRACE Gold are currently available from Metrohm. Your local Metrohm representative can assist in case of questions regarding the determination of one of the elements, or analysis in a specific matrix.
| Element | Application document |
|---|---|
| As(total) | Application Note V-210 |
| As(III) | Application Note V-211 |
| Hg | Application Note V-212 |
| Cu | Application Note V-213 |
| Pb | Application Note V-214 |
| Zn | Application Note V-215 |
| Tl | Application Note V-228 |
| Fe | Application Note V-216 |
| Ni, Co | Application Note V-217 |
| Bi | Application Note V-218 |
| Sb(III) | Application Note V-229 |
| Cr(VI) | Application Note V-230 |
Other installments in this series
This blog article was dedicated to the topic of the modified scTRACE Gold electrode and how it can be used for for the determination of heavy metal ions in drinking water. Other installments are dedicated to trace metal analysis with these solid-state electrodes:
 Share via email
Share via email
